Surgical masks are designed to be used by healthcare providers during surgical procedures. Surgical mask helps protect the sterile surgical field from being contaminated by the expelled biological particles. Surgical masks are regulated by the U.S. Food and Drug Administration (FDA) and FDA-cleared surgical masks provide high fluid resistance which is an important parameter to be considered during a surgery. Fluid-resistant surgical masks reduce exposure to high-pressure streams of blood and certain body fluids. The flat, 3-ply surgical mask is widely used by the healthcare providers in hazardous environments such as clinics, dental practices and clinical laboratory settings for its superior level of protection against dust and certain biological particles.
Healthcare professionals are required to wear a fit-tested N95 or FFP3 level respirator instead of a surgical mask for special medical procedures that generate aerosols. N95 level respirators can provide effective respiratory protection against certain liquid droplets and infectious aerosols while performing aerosol-generating medical procedures. According to the Centers for Disease Control and Prevention (CDC) guidelines, several medical procedures have been listed as aerosol-generating procedures (AGP) [1].
Aerosol generating medical procedures: Endotracheal intubation and extubation, care of intubated patient (in case of inadvertent disruption of circuit), sputum induction, bronchoscopy, open airway suctioning, high flow oxygen therapy, noninvasive ventilation, nebulizer treatment, cardiopulmonary resuscitation, manual ventilation
Certain medical procedures are not listed as aerosol generating procedures, but needs to be treated as aerosol generating procedures. An N95 or FFP3 level respirator is also required to perform these special medical procedures. Such procedures include: ENT/OMFS or other airway manipulating operative cases, tracheostomy care, endoscopy, trans-esophageal echocardiography, pulmonary function testing etc. The University of Washington Medicine recommend treating these procedures as aerosol generating procedures, as significant concern exists in these medical procedures [2]. However, they are not listed as the aerosol generating procedures by the CDC.
Aerosol generating events are the riskiest medical procedures to perform in the vicinity of pandemic flu (COVID-19) patients. Special caution should be exercised while performing these procedures with at least an N95 or FFP3 level respirator. Routine patient care or examination of a patient with unknown respiratory illness can be performed with ASTM level-3 surgical masks. However, Michael Osterholm, PhD, MPH, director of the University of Minnesota’s Center for Infectious Disease Research and Policy (CIDRAP) believes that healthcare providers should continue using an N95 particulate respirator, until there is clear evidence that N95s are not necessary for that particular medical procedures [3].
N95 respirators are regulated according to the guidelines specified by NIOSH and certain N95 respirator models are cleared by FDA to be used for surgical procedures. These FDA-cleared N95 respirators are known as the surgical N95s or healthcare particulate respirators. 3M model 1860 & 1860s, 3M model 1870, 3M model 8577, and 3M model 9541V are the most well-known surgical N95s or healthcare particulate respirators. These N95 respirator models have been designed to protect healthcare providers from certain biological particles generated from surgical processes, such as electrocautery, laser surgery etc. These respirators also help protect us from infectious diseases through its fluid resistance (ASTM F1862) from certain infectious materials, and splash and spatter of blood. These respirators also help control Mycobacterium tuberculosis exposure as per CDC guidelines.
According to World Health Organization (WHO), there are four different situations for healthcare staffs with three different levels of risk.
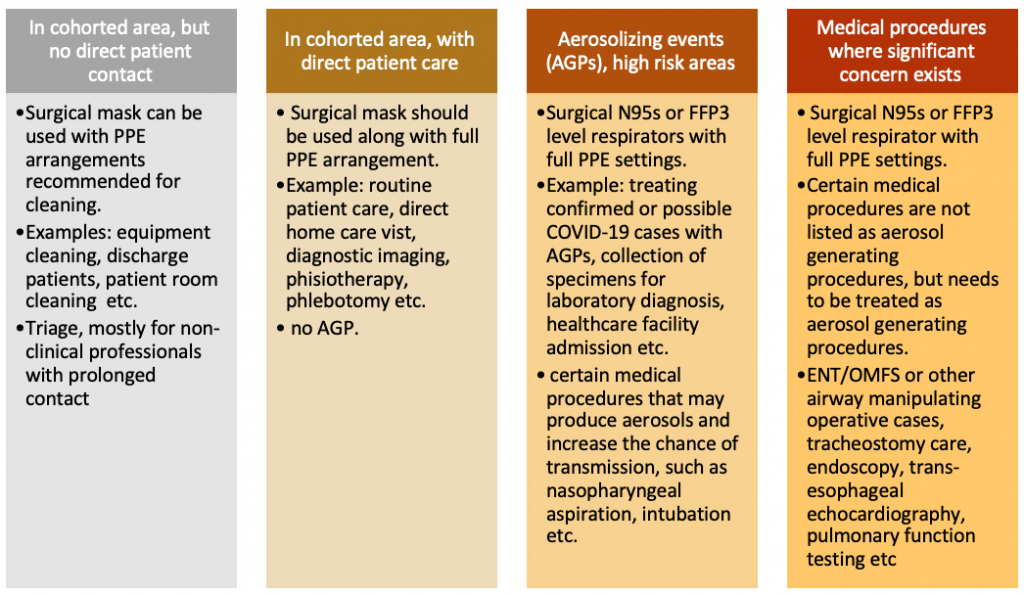
According to the World Health Organization (WHO), homemade cloth masks are not recommended for healthcare providers under any circumstances. Homemade cloth masks are not considered as part of the personal protective equipment as their effectiveness in protecting healthcare professionals is unknown.
References:
1. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html
2. https://covid-19.uwmedicine.org/Pages/default.aspx
3. https://www.cidrap.umn.edu/news-perspective/2020/03/hospitals-scramble-keep-cdc-n95-mask-guidance

