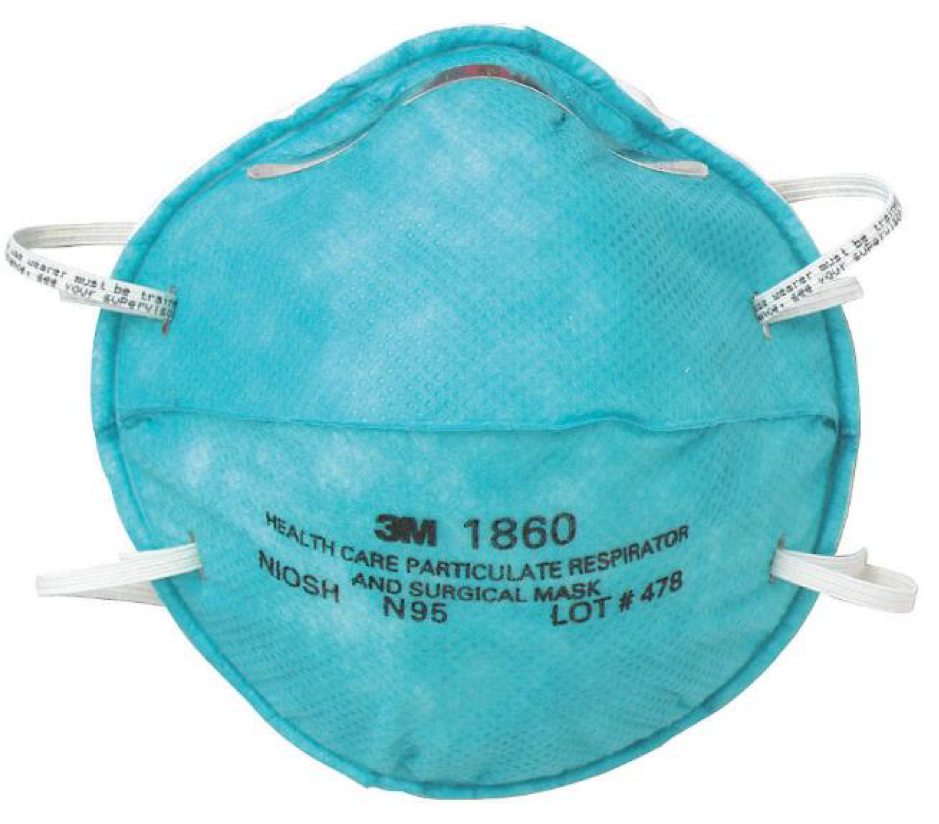
In the United States, the most well-known and commonly used particulate respirator is the N95 class medical masks (particulate respirators) which can separate at least 95% airborne particles. N95 medical mask (particulate respirator) is a respiratory protective equipment that is designed to protect the wearer from certain airborne particles. This particular type of medical mask is not generally regulated by the U.S. Food and Drug Administration (FDA), rather they are regulated by the Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH) and the Occupational Safety and Health Administration (OSHA). N95 respirators generally require clearance from the U.S. Food and Drug Administration (FDA), if the manufacturer decides to use this product for medical purposes. As a response to the COVID-19 pandemic situation, FDA cleared certain NIOSH-approved N95 respirators to be used in clinical settings during this emergency pandemic situation. N95 medical masks (particulate respirators) are widely used in healthcare facilities, clinical lab settings and certain hazardous environments where superior protection is required.
The N95 particulate respirator is intended to provide a secure facial fit and high filtration efficiency against the biological particles. The edges of the N95 particulate respirator is designed to provide a secure seal around the nose and face. The combination of nose strip, soft inner material ensures a comfortable fit that is convenient for prolonged use. Most N95 respirators are designed to fit a wide range of face shapes and sizes. N95 masks often use a two head-strap method to secure the mask to the wearer’s face: one strap goes around the back of the head under the ears, while the other strap goes on the top over the crown of the head above the ears.
N95 particulate respirators are intended to provide high fluid resistance, particulate filtration efficiency, bacterial filtration efficiency and biocompatibility. N95 medical masks also meet CDC guidelines to help control the exposure of Mycobacterium Tuberculosis (TB). N95 medical mask’s resistance against fluid also provides protection against the exposure to splash and spatter of blood and other body fluids. According to the Centers for Disease Control and Prevention (CDC) guidelines, the healthcare providers in the United States are required to wear a particulate respirator of at least class N, R or P. N95 masks are slightly more uncomfortable to wear in comparison with FFP2 or KN95 masks. However, N95 respirator fits snugger with a tighter fit as compared to KN95 or FFP2 respirators.

